Describe The Bonding In Diamond. Diamond is organised in a giant lattice structure with strong covalent bonds between carbon atoms. Diamond is made of carbon atoms that are packed in a very specific fashion.
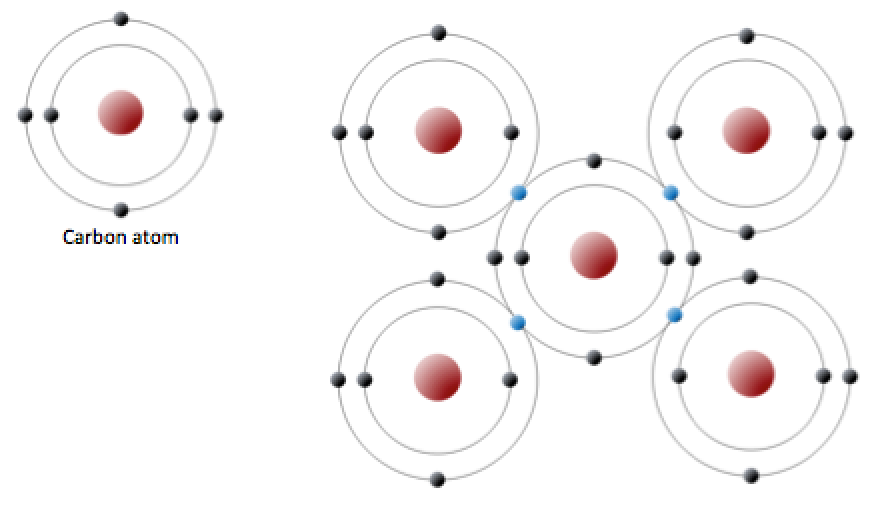
Diamond is formed of tetrahedral bonded carbon atoms with 1 carbon atom linked to 4 other carbon atoms. All of the covalent bonds in diamond are identical. Carbon atoms cannot slide.
Carbon atoms cannot slide.
We are only showing a small bit of the whole structure. Diamond is organised in a giant lattice structure with strong covalent bonds between carbon atoms. It forms a giant molecular structure. Each carbon atom forms 4 bonds.
